Tumor-on-a-chip to improve patient care
(TME On Chip)
Coordinators
Stéphanie Descroix (Physico-chimie Curie); Luc Cabel (Institut Curie, Ensemble hospitalier).
Institutions and establishments involved
Institut Curie (INSERM U830, UMR CNRS 168, UMR CNRS 144)
Context and challenges
Cancer has been the leading cause of premature death in France since 2004, with approximately 3.8 million people currently living with a cancer diagnosis. To improve patient care, it is crucial to develop new concepts and technologies. Cancer does not only affect tumor cells, but the entire tumor microenvironment (TME), composed of immune cells, fibroblasts, endothelial cells, and the extracellular matrix. This microenvironment is constantly evolving and influences cancer progression as well as response to treatments, including immunotherapies. A major challenge remains the lack of reliable patient-derived in vitro models to effectively predict response to anticancer treatments.
Scientific objectives and solutions
The Tumor-on-Chip (ToC) project aims to develop patient-derived tumor-on-chip models that can faithfully reproduce the complexity of their tumor. These devices will enable to predict the effectiveness of treatments and identify the most appropriate therapy for each patient. This work will be carried out in collaboration with interdisciplinary research teams and the Institut Curie, a reference center for breast cancer treatment in Europe.
Research program
The research program focuses on the development of ToCs from primary cells isolated from tumors of breast cancer (BC) patients, with a particular focus on :
- Triple negative breast cancers (TNBC), representing 10-15% of cases, particularly difficult to treat.
- Advanced cancers, responsible for the majority of deaths.
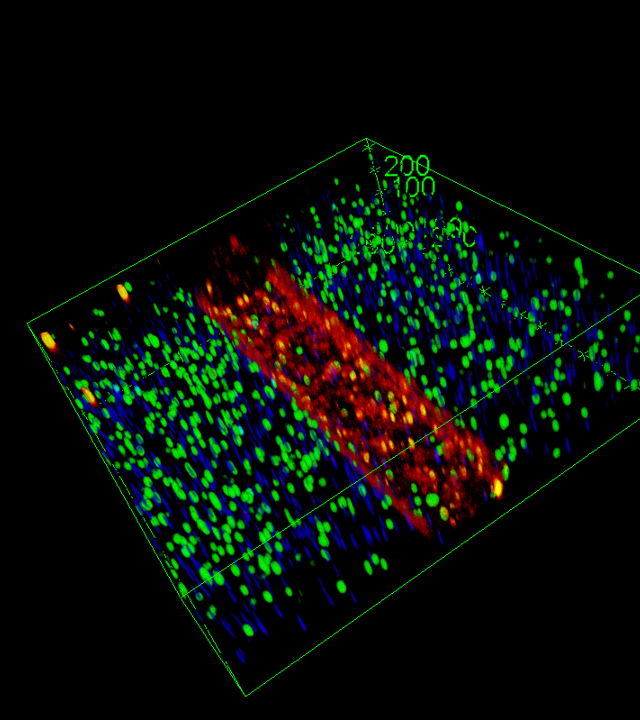
The ToC model will integrate tumor cells, cancer-associated fibroblasts (CAFs), immune cells and an endothelial vessel in a 3D matrix, with a controlled physicochemical environment. Two types of samples will be used :
- Surgical resection (75% success rate to establish a ToC).
- Biopsy, less invasive, but requiring technological developments to adapt the model to a low number of cells..
The objective is to design a robust, easy-to-use ToC capable of testing various drug combinations.
Key project milestones
Development of the ToC patient model :
Establishment of the minimum conditions necessary to reproduce the antitumor response observed in patients.
Progressive incorporation of different cell populations to assess their impact on the response to treatments.
Use of imaging and machine learning techniques to monitor cellular activity in real time (migration, interaction, cell death).
Clinical validation :
Two clinical studies will be conducted :
Observational study to assess the ability of ToC to predict patient response to treatments in early and advanced triple negative breast cancer. Approximately 80 patients will be included for each stage, with an assessment of the concordance between in vivo and in vitro tumor response.
Interventional clinical trial to select treatments based on ToC results. This study will aim to demonstrate that the therapeutic choice based on the “tumorogram” improves response rates (objective: to achieve at least 25% response, compared to 10-15% with conventional chemotherapies). Robust, easy to use and capable of testing various combinations of drugs.
The Consortium
- Team Descroix UMR 168
- Team Parrini U830
- Team Hersen, UMR 168
- Team Vignjevic, UMR 144
- Team Isambert, UMR 168
- Luc Cabel, Hôpital Curie
Plus de projets