Type 1 Diabetes on chip
(MAGIC)
Diabetes is a major public health issue, with 537 million people affected in 2021 and a projection of 783 million by 2045. Type 1 diabetes (T1D) represents 10% of this population. This chronic metabolic disease is caused by the autoimmune destruction of pancreatic beta cells, responsible for insulin secretion, leading to persistent hyperglycemia.
A therapy developed by Grenoble University Hospital, reimbursed in France since 2021, consists of transplanting pancreatic islets from cadaveric donors to restore insulin secretion in patients with T1D. This approach is effective, but 40% of patients experience allogeneic rejection or autoimmune relapse after 5 years, caused by immune T cells.
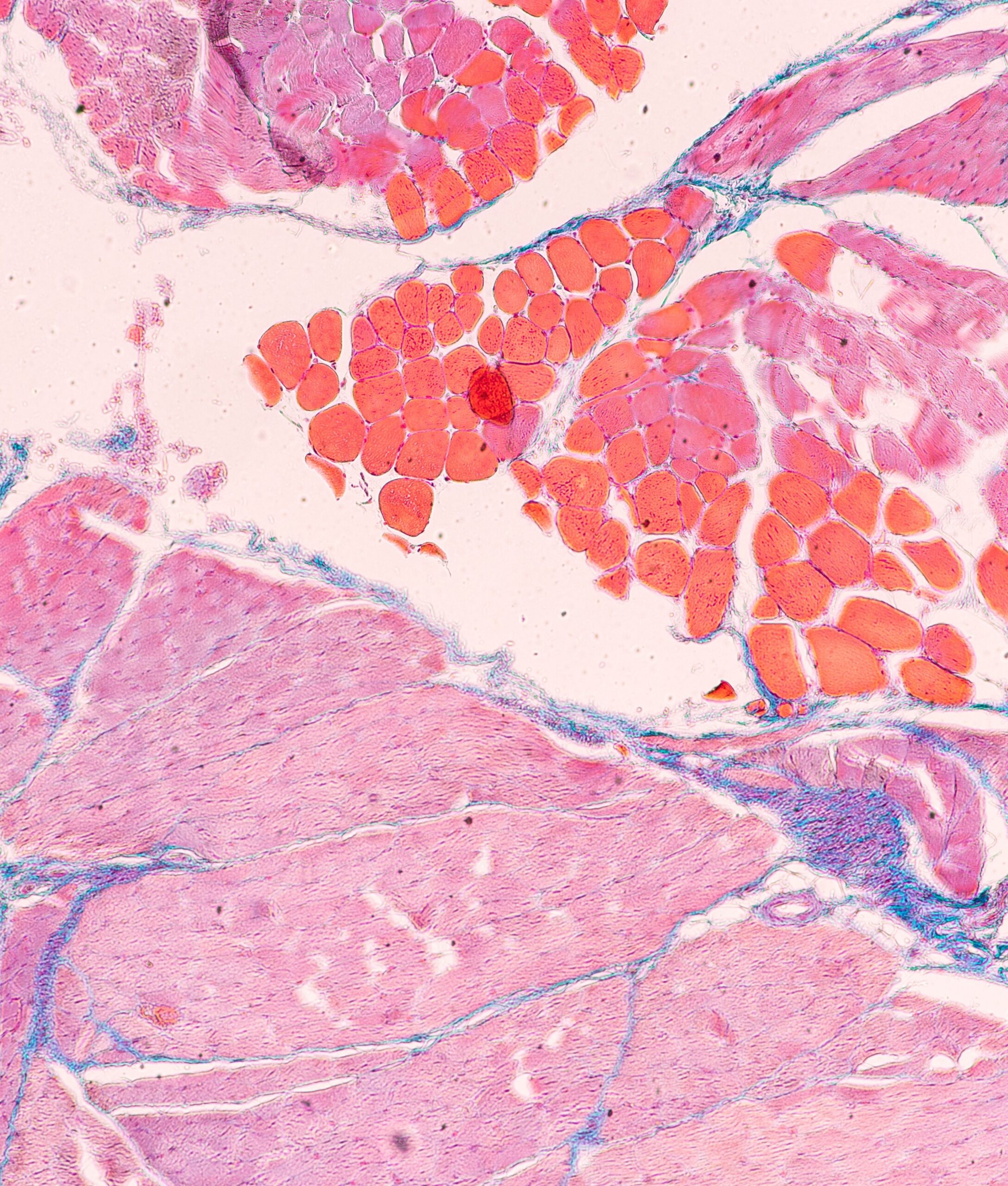
Monitoring of transplanted islets is complex because they are diffuse in the recipient’s liver, making it impossible to monitor their functionality or adapt immunosuppressive treatment.
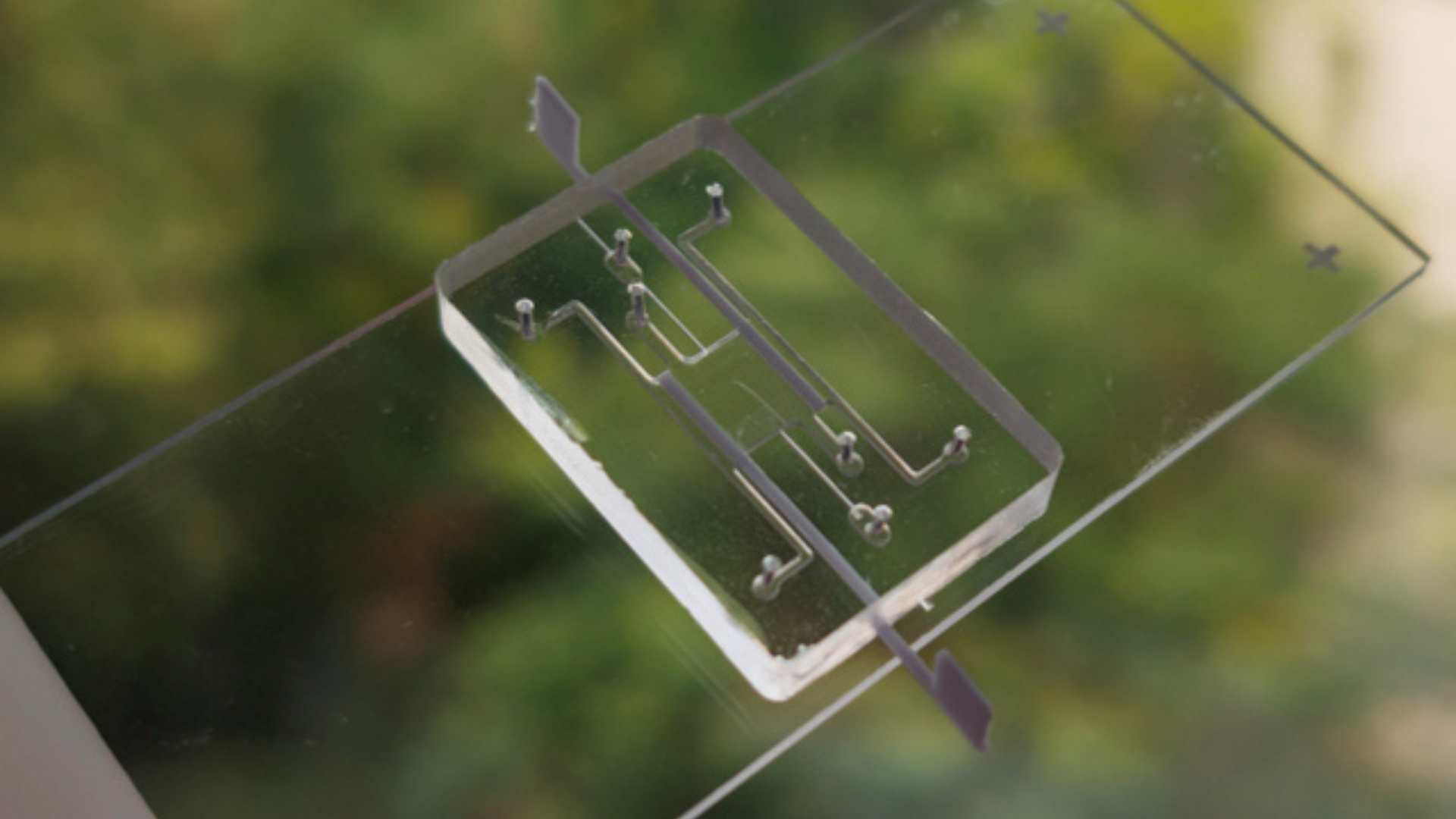
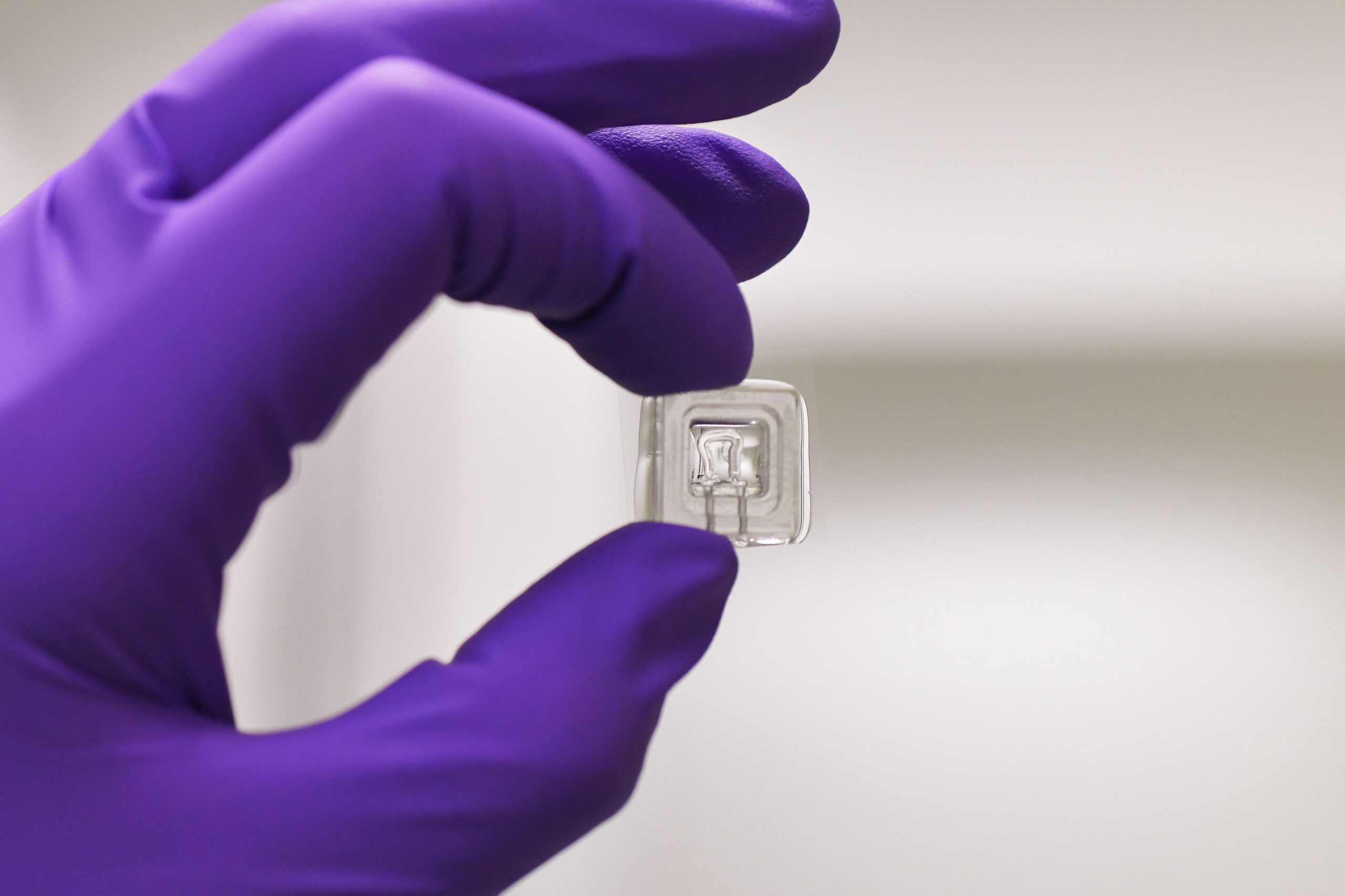
The project aims to develop vascularized islets of Langerhans on a chip (VLoC) to monitor in real time the immune response of patients who have received a pancreatic islet transplant. This technology will enable to monitor graft survival and assess its functionality by measuring insulin secretion, thus providing a predictive tool to anticipate graft rejection.
Coordinators

Department manager « Microsystems for life interaction » CEA Leti, CEA

Head of the Endocrinology, Diabetology and Nutrition Department
CHU Grenoble Alpes
Institutions and establishments involved
CEA LETI/DTBS, DRF/IRIG/BIOMICS ; CHU Grenoble Alpes
Research program
The program is based on three main axes:
- Design and development of the VLoC :
- From a frozen sample of the islets transplanted in each patient, a microfluidic device will be used to test a single islet.
- T cells isolated from the patient will be injected into the vascular network of the device to observe their impact on the survival and insulin secretion of the islet.
- Sensors and imaging technologies will be integrated to measure insulin secretion in real time, a key indicator of islet functionality.
- Clinical validation :
- A low-risk clinical study will be conducted to integrate the VLoC into the care pathway of patients with T1D.
- Twice a year, a blood sample will be taken from transplanted patients to isolate their T cells and inject them into the VLoC.
- This procedure will allow the viability of the graft to be monitored and, if necessary, the immunosuppressive treatment to be adapted. The study will involve at least 10 transplanted patients, with minimal impact on their healthcare management.
- Development of new preclinical therapeutic approaches on chips :
- The VLoC can be used to test new drugs, in particular immunosuppressants, and explore new hypotheses in immunotherapy and inflammation.
- The project also plans to develop islets of Langerhans derived from induced pluripotent stem cells (iPSCs) specific to each patient, with a view to their use as graft material.
- An innovative approach will consist in overexpressing the PD-L1 protein in the islets derived from iPSCs to deplete the T cells responsible for the autoimmune response and thus protect the grafted islets.
Ultimately, this strategy could lead to an autologous cell therapy requiring fewer immunosuppressive treatments, thus improving the quality of life of patients with T1D and generating significant medico-economic benefits.
The Consortium
Cea Leti :
- Fabrice Navarro
- Yves Fouillet
- Remco Den Dulk
- Yohann Thomas
- Joris Kaal
- Camille Laporte
Cea Irig :
- Emily Tubbs
CHU Grenoble Alpes :
- Sandrine Lablanche
Plus de projets